The Rhythm of the Atoms
If
the world's finest minds can unravel only
with difficulty the deeper workings of nature,
how could it be supposed that those workings
are merely a mindless accident, a product
of blind chance?
Paul Davies, Professor of Theoretical Physics
31
Scientists are in general agreement that,
on the basis of calculations, the Big Bang
took place about 17 billion years ago. All
the matter making up the universe was created
from nothingness but with the wonderful design
that we talked about in the first two chapters.
Nevertheless, the universe that emerged from
the Big Bang could have been much different
from the one that did emerge-ours.
For example, if the values of four fundamental
forces were different, the universe would
have consisted of only radiation and become
a tissue of light with no stars, galaxies,
human beings, or anything else. Thanks to
the extraordinary perfect balance of those
four forces, "atoms"-the building-blocks of
that which is called "matter"-came into being.
Scientists are also in general agreement
that the first two simplest elements-hydrogen
and helium-began to form during the first
fourteen seconds after the Big Bang. The elements
were formed as a result of a reduction in
the universal entropy that was causing matter
to scatter everywhere. In other words, at
first the universe was just an amassing of
hydrogen and helium atoms. If it had remained
so, again there could have been no stars,
planets, stones, soil, trees, or human beings.
It would have been a lifeless universe consisting
of only those two elements.
Carbon, the fundamental element of life,
is a much heavier element than hydrogen and
helium. How did it come into being?
Searching for an answer to this question,
scientists stumbled upon one of the most surprising
discoveries of this century.
The Structure of the
Elements
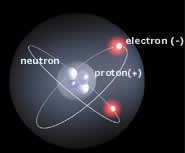 Chemistry
is a science that deals with the composition,
structure, and properties of substances and
with the transformations that they undergo.
The bedrock of modern chemistry is the periodic
table of elements. First laid out by Russian
chemist Dmitry Ivanovich Mendeleyev, the elements
in the periodic table are arranged according
to their atomic structures. Hydrogen occupies
the first place in the table because it is
the simplest of all the elements, consisting
of only one proton in its nucleus and one
electron revolving around it.
Chemistry
is a science that deals with the composition,
structure, and properties of substances and
with the transformations that they undergo.
The bedrock of modern chemistry is the periodic
table of elements. First laid out by Russian
chemist Dmitry Ivanovich Mendeleyev, the elements
in the periodic table are arranged according
to their atomic structures. Hydrogen occupies
the first place in the table because it is
the simplest of all the elements, consisting
of only one proton in its nucleus and one
electron revolving around it.
Protons are subatomic particles that carry
a positive electrical charge in the nucleus
of an atom. Helium, with two protons, occupies
the second place in the periodic table. Carbon
has six protons and oxygen has eight. All
the elements differ in the number of protons
that they contain.
Another particle present in the nucleus of
an atom is the neutron. Unlike protons, neutrons
do not carry an electrical charge: they are
neutral in other words, hence their name.
The third basic particle of which atoms are
composed is the electron, which has a negative
electrical charge. In every atom, the number
of protons and electrons is the same. Unlike
protons and neutrons however, electrons are
not located in the nucleus. Instead, they
move around the nucleus at a very high speed
that keeps the positive and negative charges
of the atom apart.
The differences in atomic structure (the
numbers of protons/electrons) are what make
the elements different from one another.
A crucial rule of (classical) chemistry is
that elements cannot be transformed into one
another. Changing iron (with twenty-six protons)
into silver (with eighteen) would require
removing eight protons from the nucleus. But
protons are bound together by the strong nuclear
force and the number of protons in a nucleus
can be changed only in nuclear reactions.
Yet all the reactions that take place under
terrestrial conditions are chemical reactions
that depend on electron exchange and that
do not effect the nucleus.
In the Middle Ages there was a "science"
called alchemy-the forerunner of modern chemistry.
Alchemists, unaware of the periodic table
or the atomic structures of the elements,
thought it was possible to transform one element
into another. (A favorite object of pursuit,
for reasons that should be apparent, was trying
to turn iron into gold.) We now know that
what the alchemists were trying to do is impossible
under normal conditions such as exist on Earth:
The temperatures and pressures required for
such a transformation to take place are too
enormous to achieve in any terrestrial laboratory.
But it is possible if you have the right place
to do it in.
And the right place, it turns out, is in
the hearts of stars.
The Universe's Alchemy
Labs: Red Giants
The temperature required to overcome the
reluctance of nuclei to change is nearly 10
million degrees Celsius. This is why "alchemy"
in the real sense takes place only in stars.
In medium-sized stars like the Sun, the enormous
energy being radiated is the result of hydrogen
being fused into helium.

Red giants are huge stars about fifty
times bigger than our sun. Deep within
these giants, an extraordinary process
takes place. |
Keeping this brief review of the chemistry
of elements in mind, let us return to the
immediate aftermath of the Big Bang. We mentioned
that only helium and hydrogen atoms existed
in the universe after the Big Bang. Astronomers
believe that solar-type stars (of which the
Sun is one) are formed as a result of nebulae
(clouds) of hydrogen and helium gas being
compressed until the hydrogen-to-helium thermonuclear
reaction gets started. So now we have stars.
But our universe is still lifeless. For life,
heavier elements-oxygen and carbon specifically-are
required. There needs to be another process
whereby hydrogen and helium can be converted
into still other elements.
The "manufacturing-plants" of these heavy
elements it turns out are the red giants-a
class of stars that are fifty times bigger
than the Sun.
Red giants are much hotter
than solar-type stars and this characteristic
enables them to do something other stars cannot:
They convert helium into carbon. Nevertheless,
even for a red giant this is not easy. As
the astronomer Greenstein says: "Even now,
when the answer (as to how they do it) is
well in hand, the method they employ seems
astonishing."32
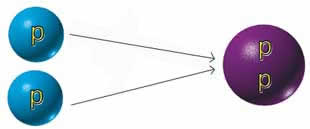
Helium's atomic weight is 2: that is, it
has two protons in its nucleus. Carbon's atomic
weight is 6. In the fantastically high temperatures
of red giants, three helium atoms are fused
into a carbon atom. This is the "alchemy"
that supplied the universe with its heavier
elements after the Big Bang.
But as we said: it's not easy. It's nearly
impossible to persuade two helium atoms to
join together and quite impossible for three.
So how do the six protons needed for carbon
get together?
It's a two-step process. First, two helium
atoms are fused into an intermediary element
with four protons and four neutrons. Next,
a third helium is added to this intermediary
element to make a carbon atom with six protons
and six neutrons.

|

|

|

|
|
Helium nucleus |
Carbon nucleus |
The extraordinarily unstable isotope
of beryllium
that is formed in red giants. |
Normal beryllium as found on Earth.
|
The intermediary element is beryllium. Beryllium
occurs naturally on Earth but the beryllium
that occurs in red giants is different in
a crucially important way: It consists of
four protons and four neutrons, whereas terrestrial
beryllium has five neutrons. "Red-giant beryllium"
is a slightly different version. It's what's
called an "isotope" in chemistry.
Now comes the real surprise. The "red-giant"
isotope beryllium turns out to be incredibly
unstable. Scientists have studied this isotope
for years and discovered that once it has
formed, it breaks down again in just 0.000000000000001
second.
How is this unstable beryllium isotope, which
forms and disintegrates in such a short time,
able to unite with a helium atom to become
a carbon atom? It is like trying to lay a
third brick on two other bricks that shoot
away from each other in 0.000000000000001
second if they chance to come atop one another,
and form a construction in this way. How does
this process take place in red giants? Physicists
scratched their heads over this puzzle for
decades without coming up with an answer.
The American astrophysicist Edwin Salpeter
finally discovered a clue to the mystery in
the concept of "atomic resonance".
Resonance and Double
Resonance
Resonance is defined as the harmony of frequencies
(vibrations) of two different materials.
A simple example from ordinary experience
will give us an idea of what physicists mean
by "atomic resonance". Imagine yourself and
a child at a playground where there are swings.
The child sits on the swing and you give him
a push to get him started. To keep the swing
moving, you have to keep pushing it from behind.
But the timing of these pushes is important.
Each time the swing approaches you, you have
to apply the force of the push just at the
right moment: when the swing is at the highest
point of its motion towards you. If you push
too soon, the result is a collision that disturbs
the rhythmic momentum of the swing; if you
push too late, the effort is wasted because
the swing is already moving away from you.
In other words, the frequency of your pushes
must be in harmony with the frequency of the
swing's approaches to you.
Physicists refer to such
a "harmony of frequencies" as "resonance".
The swing has a frequency: for example it
reaches you every 1.7 seconds. Using your
arms you push it every 1.7 seconds. Of course
if you want, you can change the frequency
of the swing's motion, but if you do, you
have to change the frequency of the pushes
as well, otherwise the swing will not swing
right.33
Just as two or more moving
bodies can resonate, resonance can also occur
when one moving body causes motion in another.
This type of resonance is often seen in musical
instruments and is called "acoustic resonance".
It can occur, for example, among two finely-tuned
violins. If one of these violins is played
in the same room as the other, the strings
of the second will vibrate and produce a sound
even though nobody is touching it. Because
both instruments have been precisely tuned
to the same frequency, a vibration in one
causes a vibration in the other.34
The resonances in these two examples are
simple ones and are easy to keep the track
of. There are other resonances in physics
that are not simple at all and in the case
of atomic nuclei, the resonances can be quite
complex and sensitive.
Every atomic nucleus has
a natural energy level that physicists have
been able to identify after lengthy study.
These energy levels are quite different from
one another but a few rare instances of resonance
between atomic nuclei have been observed.
When such resonance occurs, the motions of
the nuclei are in harmony with one another
like our examples of the swing and violin.
The important point of this is that the resonance
expedites nuclear reactions that can affect
the nuclei.35
Investigating how carbon was made by red
giants, Edwin Salpeter suggested that there
must be a resonance between helium and beryllium
nuclei that facilitated the reaction. This
resonance, he said, made it easier for helium
atoms to fuse into beryllium and this could
account for the reaction in red giants. Subsequent
research however failed to support this idea.

Fred Hoyle was the first to discover
the amazing equilibrium of nuclear reactions
taking place in red giants. Although an
atheist, Hoyle admitted that this balance
could not be explained by chance and that
it was a deliberate arrangement. |
Fred Hoyle was the second astronomer to address
this question. Hoyle took Salpeter's idea
a step further, introducing the idea of "double
resonance". Hoyle said that there had to be
two resonances: one that caused two heliums
to fuse into beryllium and one that caused
the third helium atom join this unstable formation.
Nobody believed Hoyle. The idea of such a
precise resonance occurring once was hard
enough to accept; that it should occur twice
was unthinkable. Hoyle pursued his research
for years and in the end he proved that his
idea was right: there really was a double
resonance taking place in the red giants.
At the exact moment two helium atoms resonated
in union, a beryllium atom appeared in the
0.000000000000001 second needed to produce
carbon. George Greenstein describes why this
double resonance is indeed an extraordinary
mechanism:
There are
three quite separate structures in this story-helium,
beryllium, and carbon-and two quite separate
resonances. It is hard to see why these nuclei
should work together so smoothly…Other nuclear
reactions do not proceed by such a remarkable
chain of lucky breaks…It is like discovering
deep and complex resonances between a car,
a bicycle, and a truck. Why should such disparate
structures mesh together so perfectly? Upon
this our existence, and that of every life
form in the universe, depends.36
In the years that followed
it was discovered that other elements like
oxygen are also formed as a result of such
amazing resonances. A zealous materialist,
Fred Hoyle's discovery of these "extraordinary
transactions" forced him to admit in his book
Galaxies, Nuclei and Quasars, that such double
resonances had to be the result of design
and not coincidence. 37
In another article he wrote:
If you wanted
to produce carbon and oxygen in roughly equal
quantities by stellar nucleosynthesis, these
are the two levels you would have to fix,
and your fixing would have to be just about
where these levels are actually found to be…A
commonsense interpretation of the facts suggests
that a super intellect has monkeyed with physics,
as well as chemistry and biology, and that
there are no blind forces worth speaking about
in nature. The numbers one calculates from
the facts seem to me so overwhelming as to
put this conclusion almost beyond question.38
Hoyle declared that the inescapable conclusion
of this plain truth should not go unnoticed
by other scientists.
I do not
believe that any scientist who examined the
evidence would fail to draw the inference
that the laws of nuclear physics have been
deliberately designed with regard to the consequences
they produce inside the stars.39
This plain truth was
expressed in the Qur'an 1,400 years ago. Allah
indicates the harmony in creation of the heavens
in the verse: Do you not see how Allah created
seven heavens in harmony… (Surah Nuh: 15)
A Lesser Alchemy Lab:
The Sun
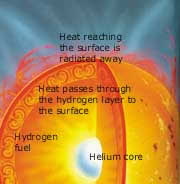
The sun is a giant nuclear reactor that
constantly transforms atoms of hydrogen
into helium and produces heat in the process.
What is crucial to this process however
is the incredible precision with which
these reactions are balanced within the
sun. The slightest change in any of the
forces governing these reactions would
result in their failure or in a catastrophic
runaway explosion. |
The conversion of helium into carbon described
above is the alchemy of red giants. In smaller
stars like our sun, a simpler sort of alchemy
takes place. The sun converts hydrogen into
helium and this reaction is the source of
its energy.
This reaction is no less essential for us
to exist than are the reactions in the red
giants. Moreover, the sun's nuclear reaction
is also a designed process, just like the
one in red giants.
Hydrogen, the input element for this reaction,
is the simplest element in the universe for
its nucleus consists of a single proton. In
a helium nucleus, there are two protons and
two neutrons. The process taking place in
the sun is the fusion of four hydrogen atoms
into one helium atom.
An enormous amount of energy is released
during this process. Nearly all the thermal
and light energy reaching Earth is the result
of this solar nuclear reaction.
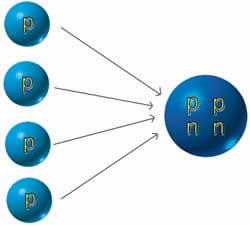
| Single-proton
hydrogen nuclei |
Helium
nucleus with two protons and two nueutrons |
THE CRITICAL REACTION
IN THE SUN
1) Above: Four
hydrogen atoms in the sun join together to
form a single helium atom.
2) Below1: This
is a two-step process. First two hydrogen
atoms fuse forming a deuteron. This transformation
is a slow one and is what keeps the sun burning
constantly.
3) Below2: If
the strong nuclear force were just a little
bit stronger, a di-proton would be formed
instead of a deuteron. Such a reaction however
cannot be sustained for any length of time:
a runaway catastrophic explosion would occur
in just a few seconds.
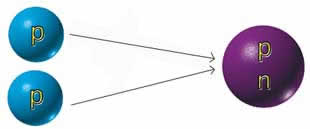
| Single-proton
hydrogen nuclei |
Deutron
nucleus with one proton and one neutron
|
| Single-proton
hydrogen nuclei |
Di-proton
nucleus with two proton |
Like the reactions taking place in red giants,
this solar nuclear reaction turns out to involve
a number of unexpected aspects without which
it could not take place. You can't simply
jam four hydrogen atoms together and turn
them into helium. To make this happen, a two-step
process is required, paralleling the one taking
place in red giants. In the first step, two
hydrogen atoms combine to form an intermediary
nucleus called deuteron consisting of one
proton and one neutron.
What force could be great enough to produce
a deuteron by jamming two nuclei together?
This force is the "strong nuclear force",
one of the four fundamental forces of the
universe mentioned in the previous section.
This is the most powerful physical force in
the universe and is billions of billions of
billions of billions times stronger than the
gravitational force. Nothing but this force
could unite two nuclei like this.
Now the really curious
thing about all this is that research shows
that, strong as it is, the strong nuclear
force is just barely strong enough to do what
it does. If it were even slightly weaker than
it is, it would not be able to unite the two
nuclei. Instead, two protons nearing each
other would repel each other immediately and
the reaction in the sun fizzle out before
it ever began. In other words, the sun would
not exist as an energy-radiating star. Concerning
this, George Greenstein says: "Had the strong
force had been only slightly less strong,
the light of the world would have never been
lit."40
What, on the other hand, if the strong nuclear
force were stronger? To answer that, we first
have to look at the process of converting
two hydrogen atoms into a deuteron in a little
more detail. First, one of the protons is
stripped of its electrical charge and becomes
a neutron. This neutron forms a deuteron by
uniting with a proton. The force causing this
unification is the "strong nuclear force";
the force that converts a proton into a neutron
on the other hand is a different one and is
called the "weak nuclear force". It is weak
only by comparison however and it takes about
ten minutes to make the conversion. At the
atomic level, this is an immensely long time
and it has the effect of slowing down the
rate at which the reaction in the sun takes
place.
Let us now return to our question: What would
happen if the strong nuclear force were stronger?
The answer is that the reaction in the sun
would be changed dramatically because the
weak nuclear force would be eliminated from
the reaction.
If the strong nuclear force were any stronger
than it is, it would be able to fuse two protons
to one another immediately and without having
to wait ten minutes for a proton to be converted
into a neutron. As a result of this reaction,
there would be one nucleus with two protons
instead of a deuteron. Scientists call such
a nucleus a "di-proton". It is a theoretical
particle however insofar as it has never been
observed to occur naturally. But if the strong
nuclear force were much stronger than it is,
then there would be real di-protons in the
sun. So what? Well by getting rid of the proton-to-neutron
conversion, we would be eliminating the "throttle"
that keeps the sun's "engine" running as slowly
as it does. George Greenstein explains what
the result of that would be:
The Sun
would change because the first stage in the
formation of helium would no longer be the
formation of the deuteron. It would be the
formation of the di-proton. And this reaction
would not involve the transformation of a
proton into a neutron at all. The role of
the weak force would be eliminated, and only
the strong force would be involved…and as
a result the Sun's fuel would suddenly become
very good indeed. It would become so powerful,
so ferociously reactive, that the Sun and
every other star like it would instantaneously
explode.41
The explosion of the sun would cause the
world and everything on it to burst into flames,
burning our blue planet to a crisp in a few
seconds. Because the strong nuclear force
is precisely fine-tuned to be neither too
strong nor too weak, the sun's nuclear reaction
is slowed down and the star has been able
to radiate light and energy for billions of
years. This precise tuning is what makes it
possible for mankind to live. If there were
even the slightest deviation in this arrangement,
the stars (including our sun) would not exist
or if they did, they would explode in a short
time.
In other words the structure of the sun is
neither accidental nor unintentional. Quite
the contrary: Allah has created the sun for
people to live, as expressed in the verse:
The sun and the moon
follow courses (exactly) computed. (Surat
ar-Rahman: 5)
Protons and Electrons
So far we have been examining matters concerned
with forces that affect atomic nuclei. There
is another important equilibrium in the atom
that we must consider: the balance between
its nucleus and electrons.
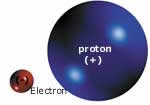
Both the mass and the volume of a
proton are incomparably larger than those
of an electron but, strangely enough,
these two particles have equal (though
opposite) electrical charges. Because
of this fact, atoms are electrically neutral.
|
Put in its simplest terms, electrons revolve
around the nucleus. The reason for this is
electrical charge. Electrons have a negative
charge and protons have a positive charge.
Opposite charges attract, so an atom's electrons
are drawn towards the nucleus. But the electrons
are also moving at an enormous speed which
would, under normal conditions, cause them
to shoot away from the nucleus. These two
forces (attraction and motion away) are balanced
so that the electrons move in orbits around
the nucleus.
Atoms are also balanced in terms of their
electric charges: the number of orbiting electrons
is the same as the number of protons in the
nucleus. (For example, oxygen has eight protons
and eight electrons.) In this way the electrical
force of an atom is balanced and the atom
is electrically neutral.
So far, so much basic chemistry. However
there is a point in this seemingly simple
structure that is overlooked by many. A proton
is much bigger than an electron in terms of
both size and weight. If an electron were
the size of a walnut, a proton would be about
the size of a man. Physically, they are quite
dissimilar.
But their electrical charges are the same
size!
Although their electrical charges are opposite
(electrons negative, protons positive) they
are also equal. There is no obvious reason
why this should be so. Conceivably (and "logically")
an electron ought to carry a much smaller
charge because it is so much smaller.
But if that were true, then what would happen?
What would happen is that every atom in the
universe would be positively charged instead
of being electrically neutral. And because
like charges repel, every atom in the universe
would try and repel every other atom. Matter
as we know it could not exist.
What would happen if it suddenly became true
now? What would happen if every atom were
to start repelling every other?
Quite extraordinary things would happen.
Let us begin with the changes that would occur
in your body. The moment this change occurred,
your hands and your arms holding this book
would shatter at once. And not just your hands
and arms but also your body, your legs, your
eyes, your teeth-every part of your body would
explode in a split second.
The room you sit in and the world around
you would explode in a moment. All the seas,
mountains, the planets in the solar system,
and all the stars and galaxies in the universe
would shatter into atomic dust. And there
would never again be anything in the universe
to observe. The universe would become a mass
of disorganized atoms pushing each other around.
By how much would the sizes of the electrical
charges of protons and electrons have to differ
in order for this dreadful thing to happen?
One percent? A tenth of one percent? George
Greenstein addresses this question in The
Symbiotic Universe:
Small things
like stones, people, and the like would fly
apart if the two charges differed by as little
as one part in 100 billion. Larger structures
like the Earth and the Sun require for their
existence a yet more perfect balance of one
part in a billion billion.42
Here is yet another precisely-tuned
equilibrium that proves that the universe
is intentionally designed and created for
a particular purpose. As John D. Barrow and
Frank J. Tipler maintain in their book "The
Anthropic Cosmological Principle", "there
is a grand design in the Universe that favours
the development of intelligent life."43
Of course every design
proves the existence of a conscious "designer".
That is Allah alone, "Lord of all the worlds",
described in the Qur'an as the only Power
Who created the universe from nothingness,
and designed and fashioned it as He willed.
As stated in the Qur'an, "He built the heaven,
He raised its vault high and made it level."
(Surat an-Nazi'at: 27-28)
Thanks to the extraordinary balances that
we have seen in this chapter, matter is able
to remain stable and this stability is evidence
of the perfection of Allah's creation as revealed
in the Qur'an:
Among His Signs is
that heaven and earth hold firm by His command.
(Surat ar-Rum: 25)
