| The Design in Light
 That
the radiation from the sun (and from many sequence
stars) should be concentrated into a minuscule
band of the electromagnetic spectrum which provides
precisely the radiation required to maintain life
on earth is very remarkable. That
the radiation from the sun (and from many sequence
stars) should be concentrated into a minuscule
band of the electromagnetic spectrum which provides
precisely the radiation required to maintain life
on earth is very remarkable.
Ian Campbell, British Physicist 65
The sun is probably the one thing we see most
often throughout our lives. Whenever
we raise our sight to the sky during the day,
we can see its dazzling light. If someone were
to come up and ask "What good is the sun? we would
probably reply without even a thought that the
sun gives us light and heat. That answer, although
a bit superficial, would be correct.
But does the sun just "happen" to radiate light
and heat for us? Is it accidental and unplanned?
Or is the sun specially designed for us? Could
this great ball of fire in the sky be a gigantic
"lamp" that was created so as to meet our exact
needs?
Recent research indicates that the answer to
the last two questions is "yes". "Yes" because
in sunlight there is a design that inspires amazement.
The Right Wavelength
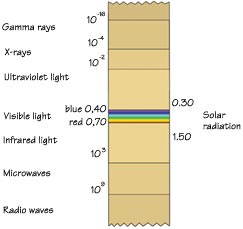
THE DIFFERENT WAVELENGTHS OF ELECTROMAGNETIC
RADIATION
The stars and other sources of light in the
universe do not all give out the same kind
of radiation. Instead, they radiate energy
with a broad range of wavelengths. Gamma rays,
which have the shortest wavelengths, are just
1/1025 the length of the longest radio waves.
Strangely enough, nearly all of the radiation
emitted by the sun falls into a single band
that is also 1/1025 of the whole spectrum.
The reason, is that the only kinds of radiation
that are necessary and fit for life fall in
this narrow band. |
Both light and heat are different manifestations
of electromagnetic radiation. In all its manifestations,
electromagnetic radiation moves through space
in waves similar to those created when a stone
is thrown into a lake. And just as the ripples
created by the stone may have different heights
and the distances between them may vary, electromagnetic
radiation also has different wavelengths.
The analogy shouldn't be taken too far however
because there are huge differences in the wavelengths
of electromagnetic radiation. Some are several
kilometers long while others are shorter than
a billionth of a centimeter and the other wavelengths
are to be found in a smooth, unbroken spectrum
everywhere in between. To make things easier,
scientists divide this spectrum up according to
wavelength and they assign different names to
different parts of it. The radiation with the
shortest wavelength (one-trillionth of a centimeter)
for example is called "gamma rays": these rays
pack tremendous amounts of energy. The longest
wavelengths are called "radio waves": they can
be several kilometers long but carry very little
energy. (One result of this is that radio waves
are quite harmless to us while exposure to gamma
rays can be fatal.) Light is a form of electromagnetic
radiation that lies between these two extremes.
The first thing to be noticed about the electromagnetic
spectrum is how broad it is: the longest wavelength
is 1025 times the size of the shortest
one. Written out in full, 1025 looks
like this:
10,000,000,000,000,000,000,000,000
A number that big is pretty meaningless by itself.
Let's make a few comparisons.
For example, in 4 billion years (the estimated
age of the earth) there are about 1017
seconds. If you wanted to count from 1 to 1025
and did so at the rate of one number a second
nonstop, day and night, it would take you 100
million times longer than the age of the earth!
If we were to build a pile of 1025
playing cards, we would end up with a stack stretching
halfway across the observable universe.
This is the vast spectrum over which the different
wavelengths of the universe's electromagnetic
energy extend. Now the curious thing about this
is that the electromagnetic energy radiated by
our sun is restricted to a very, very narrow section
of this spectrum. 70% of the sun's radiation has
wavelengths between 0.3 and 1.50 microns and within
that narrow band there are three types of light:
visible light, near-infrared light, and ultraviolet
light.
Three kinds of light might seem quite enough
but all three combined make up an almost insignificant
section of the total spectrum. Remember our 1025
playing cards extending halfway across the universe?
Compared with the total, the width of the band
of light radiated by the sun corresponds to just
one of those cards!
Why should sunlight be limited to such a narrow
range?
The answer to that question is crucial because
the only radiation that is capable of supporting
life on earth is the kind that has wavelengths
falling within this narrow range.
In Energy and the Atmosphere,
the British physicist Ian Campbell addresses this
question and says "That the radiation from the
sun (and from many sequence stars) should be concentrated
into a minuscule band of the electromagnetic spectrum
which provides precisely the radiation required
to maintain life on earth is very remarkable."
According to Campbell, this situation is "staggering".66
Let us now examine this "staggering design of
light" more closely.
From Ultraviolet to Infrared
We said that there was a range of 1:1025
in the sizes of the longest and shortest electromagnetic
wavelengths. We also said that the amount of energy
that was carried depended upon the wavelength:
shorter wavelengths pack more energy than longer
ones. Another difference has to do with how radiation
at different wavelengths interacts with matter.
The shortest forms of radiation are called (in
increasing order of wavelength) "gamma rays",
"X-rays", and "ultraviolet light". They have the
ability to split atoms because they are so highly
energized. All three can cause molecules-especially
organic molecules-to break up. In effect, they
tear matter apart at the atomic or molecular level.
Radiation with wavelengths longer than visible
light begins at infrared and extends up to radio
waves. Its impact upon matter is less serious
because the energy it conveys is not as great.
The "impact upon matter" that we spoke of has
to do with chemical reactions. A significant number
of chemical reactions can take place only if energy
is added to the reaction. The energy required
to start a chemical reaction is called its "energy
threshold". If the energy is less than this threshold,
the reaction will never start and if it is more,
it is of no good: in either case, the energy will
have been wasted.
In the whole electromagnetic spectrum, there
is just one little band that has the energy to
cross this threshold exactly. Its wavelengths
range between 0.70 microns and 0.40 microns and
if you'd like to see it, you can: just raise your
head and look around-it's called "visible light".
This radiation causes chemical reactions to take
place in your eyes and that is why you are able
to see.
The radiation known as "visible
light" makes up 41% of sunlight even though it
occupies less than 1/1025 of the whole
electromagnetic spectrum. In his famous article
"Life and Light", which appeared in Scientific
American, the renowned physicist George Wald considered
this matter and wrote "the radiation that is useful
in prompting orderly chemical reactions comprises
the great bulk of that of our sun."67
That the sun should radiate light so exactly right
for life is indeed an extraordinary example of
design.
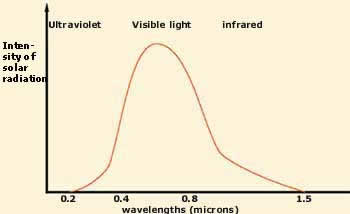 Nearly all of the sun's radiation is
restricted to a narrow band of wavelengths
ranging from 0.3 to 1.50 microns. This band
encompasses near ultraviolet, visible, and
infrared light.
Nearly all of the sun's radiation is
restricted to a narrow band of wavelengths
ranging from 0.3 to 1.50 microns. This band
encompasses near ultraviolet, visible, and
infrared light. |
Is the rest of the light the sun radiates good
for anything?
When we look at this part of
the light we see that a large part of solar radiation
falling outside the range of visible light is
in the section of the spectrum called "near infrared".
This begins where visible light ends and again
occupies a very small part of the total spectrum-less
than 1/1025.68
Is infrared light good for anything? Yes, but
this time it's no use to look around because you
can't see it with the naked eye. However you can
easily feel it: the warmth you feel on your face
when you look up on a bright sunny summer or spring
day is caused by infrared radiation coming from
the sun.
The sun's infrared radiation is what carries
the thermal energy that keeps Earth warm. It too
is as essential for life as visible light is.
And the fascinating thing is that our sun was
apparently created just to serve for these two
purposes, because these two kinds of light make
up the greatest part of sunlight.
And the third part of sunlight? Is that of any
benefit?
You can bet on it. This is
"near ultraviolet light" and it makes up the smallest
fraction of sunlight. Like all ultraviolet light,
it is highly energized and it can cause damage
to living cells. The sun's ultraviolet light however
is the "least harmful" kind since it is closest
to visible light. Although overexposure to solar
ultraviolet light has been shown to cause cancer
and cellular mutations, it has one vital benefit:
the ultraviolet light concentrated in such a miniscule
band 69
is needed for the synthesis of vitamin D in humans
and other vertebrates. (Vitamin D is necessary
for the formation and nourishment of bone: without
it, bones become soft or malformed, a disease
called rickets that occurs in people deprived
of sunlight for great lengths of time.)
In other words, all the radiation emitted by
the sun is essential to life: none of it is wasted.
The amazing thing is that all this radiation is
limited to a 1/1025 interval of the
whole electromagnetic spectrum yet it is sufficient
to keep us warm, see, and allow all the chemical
reactions necessary for life to take place.
Even if all the other conditions necessary for
life and mentioned elsewhere in this book existed,
if the light radiated by the sun fell into any
other part of the electromagnetic spectrum, there
could be no life on Earth. It is certainly impossible
to explain the fulfillment of this condition having
a probability of 1 in 1025 with a logic
of coincidence.
And if all this were not enough, light does something
else: it keeps us fed, too!
Photosynthesis and Light
Photosynthesis is a chemical process whose name
almost everyone who's ever gone to school will
be familiar with. Most people however fail to
realize how vitally important this process is
for life on Earth or what a mystery its workings
are.
First let's brush off our high-school chemistry
and take a look at the formula for the photosynthesis
reaction:
6H2O + 6CO2 +Sunlight -->
C6H12O6 + 6O2
Glucose
Translated into words this means: Water and carbon
dioxide and sunlight produces glucose and oxygen.
To be more exact what is happening in this chemical
reaction is that six molecules of water (H2O)
combine with six molecules of carbon dioxide (CO2)
in a reaction that is energized by sunlight. When
the reaction is complete, the result is a single
molecule of glucose ( C6H12O6),
a simple sugar that is a fundamental element of
nutrition-, and six molecules of gaseous oxygen
(O2). The source of all nutriments
on our planet, glucose contains a great deal of
energy.
Simple though this reaction may look, it is in
fact incredibly complex. There is only one place
where it occurs: in plants. The plants of this
world produce the basic food for all living things.
Every other living thing is ultimately nourished
in one way or another by glucose. Herbivorous
animals eat the plants themselves and carnivorous
animals eat plants and/or other animals. Human
beings are no exception: our energy is derived
from the food we eat and comes from the same source.
Every apple, potato, chocolate, or steak or anything
else you eat is supplying you with energy that
came from the sun.
But photosynthesis is important for another reason.
The reaction has two products: in addition to
glucose, it also releases six molecules of oxygen.
What's happening here is that plants are continuously
cleaning up an atmosphere that is constantly being
"polluted" by air-breathing creatures-human beings
and animals, whose energy is derived from combustion
in oxygen, a reaction that produces carbon dioxide.
If plants didn't release oxygen, the oxygen-breathers
would eventually use up all the free oxygen in
the atmosphere and that would be the end of them.
Instead, the oxygen in the atmosphere is constantly
being replenished by plants.

For hundreds of millions of years, plants
have been busy doing something no laboratory
has ever been able to duplicate: Using sunlight,
the produce food. A crucial condition for
this extraordinary transformation however
is that the light that the plants receive
must be precisely right for photosynthesis
to take place. |
Without photosynthesis, plant life could not
exist; and without plant life, there would be
no animal or human life. This marvelous chemical
reaction, which has never been duplicated in any
laboratory, is taking place deep in the grass
you step on and in trees you may not even notice.
It once occurred in the vegetables on your dinner
plate. It is one of the fundamental processes
of life.
The interesting thing is what a carefully-designed
process photosynthesis is. When we study it, we
can't help but observe that there is a perfect
balance between plant photosynthesis and the energy
consumption of oxygen-breathers. Plants supply
glucose and oxygen. Oxygen-breathers burn the
glucose in the oxygen in their cells to get energy
and they release carbon dioxide and water (in
effect, they're reversing the photosynthesis reaction)
that the plants use to make more glucose and oxygen.
And so it goes on, a continuous cycle that is
called the "carbon cycle" and it is powered by
the energy of the sun.
In order to see how perfectly-created this cycle
truly is, let us focus our attention on just one
of its elements for the moment: the sunlight.
In the first part of this chapter we looked at
sunlight and found that its radiation components
were specially tailored to allow life on Earth.
Could sunlight also be deliberately tailored for
photosynthesis as well? Or are plants flexible
enough so that they can perform the reaction no
matter which kind of light reaches them?
The American astronomer George Greenstein discusses
this in The Symbiotic Universe:
Chlorophyll
is the molecule that accomplishes photosynthesis...
The mechanism of photosynthesis is initiated by
the absorption of sunlight by a chlorophyll molecule.
But in order for this to occur, the light must
be of the right color. Light of the wrong color
won't do the trick.
A good analogy is that of a television
set. In order for the set to receive a given channel
it must be tuned to that channel; tune it differently
and the reception will not occur. It is the same
with photosynthesis, the Sun functioning as the
transmitter in the analogy and the chlorophyll
molecule as the receiving TV set. If the molecule
and the Sun are not tuned to each other-tuned
in the sense of colour- photosynthesis will not
occur. As it turns out, the sun's color is just
right.70
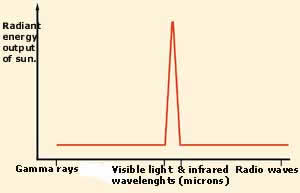 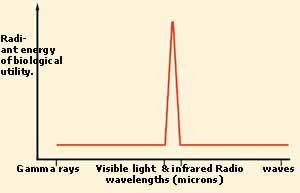
THE FITNESS OF SUNLIGHT AND CHLOROPHYLL
Plants are able to perform photosynthesis
because the chlorophyll molecules in their
cells are sensitive to sunlight. But chlorophyll
is only able to use a very limited range
of light wavelengths and those are the wavelengths
that the sun radiates the most. What is
even more interesting is that this interval
corresponds to just 1/1025 of
the whole electromagnetic spectrum.
In the two graphs above, the extraordinary
fitness between sunlight and chlorophyll
can be seen. In the upper chart is the distribution
of the light emitted by the sun. In the
lower one is the light under which photosynthesis
will work. The fact that these two curves
are almost identical is an indication of
how perfectly designed visible light is.
< |
In the last chapter we drew attention to the
error inherent in the idea of the adaptability
of life. Some evolutionists hold that "if conditions
had been different, life would have evolved to
be perfectly in harmony with them as well". Thinking
superficially about photosynthesis and plants,
one could come to a similar conclusion: "If sunlight
were different, plants would have just evolved
according to that." But this is in fact impossible.
Although he's an evolutionist himself, George
Greenstein admits this:
One might think
that a certain adaptation has been at work here:
the adaptation of plant life to the properties
of sunlight. After all, if the Sun were a different
temperature could not some other molecule, tuned
to absorb light of a different colour, take the
place of chlorophyll? Remarkably enough the answer
is no, for within broad limits all molecules absorb
light of similar colours. The absorption of light
is accomplished by the excitation of electrons
in molecules to higher energy states, and the
same no matter what molecule you are discussing.
Furthermore, light is composed of photons, packets
of energy and photons of the wrong energy simply
can not be absorbed… As things stand in reality,
there is a good fit between the physics of stars
and that of molecules. Failing this fit, however,
life would have been impossible.71
What Greenstein is saying briefly is this: No
plant can only perform photosynthesis except within
a very narrow range of light wavelengths. And
that range corresponds exactly to the light given
out by the sun.
The harmony between stellar and molecular physics
that Greenstein refers to is a harmony too extraordinary
ever to be explained by chance. There was only
one chance in 1025 of the sun's providing
just the right kind of light necessary for us
and that there should be molecules in our world
that are capable of using that light. This perfect
harmony is unquestionably proof of intentional,
deliberate design.
In other words, there is a single Creator, the
Ruler of starlight and of the molecules of plants
Who has created all these things in harmony with
one other, exactly as is revealed in the Qur'an:
He is Allah- the Creator,
the Maker, the Giver of Form. To Him belong the
Most Beautiful Names. Everything in the heavens
and earth glorifies Him. He is the Almighty, the
All Wise. (Surat al-Hashr: 24)
The Light of Your Eyes
We have seen how the light coming to us from
the sun consists of just three narrow bands of
the electromagnetic spectrum:
1) Infrared light, whose wavelengths are longer
than visible light and which keeps Earth warm.
2) A small amount of ultraviolet light, whose
wavelengths are shorter than visible light and
which is necessary for the synthesis of vitamin
D among other things.
3) Visible light, which makes vision possible
and supports plant photosynthesis.
The existence of a range of
"visible light" is as important for the support
of biological vision as it is for photosynthesis.
The reason is that it is impossible for a biological
eye to see any band of the spectrum outside that
of visible light and a very small section of near
infrared.
To explain why this should be so, we first need
to understand how vision takes place. It begins
with particles of light called "photons" passing
through the pupil of eye and falling onto the
surface of the retina located at the back of the
eye. The retina contains cells that are light-sensitive.
They are so sensitive that each can recognize
when even a single photon strikes it. The photon's
energy activates a complex molecule called "rhodopsine",
large quantities of which are contained in these
cells. The rhodopsine in turn activates other
cells and those activate still others in turn.72
Eventually an electrical current is generated
and this is carried to the brain by the optic
nerves.
The first requirement for this system to work
is that the retina cell must be able to recognize
when a photon strikes it. For that to happen,
the photon must carry an exact amount of energy:
if it is too much or too less, it won't activate
the formation of rhodopsine. Changing the size
of the eye makes no difference: the crucial thing
is the harmony between the size of the cell and
the wavelengths of the photons coming in.
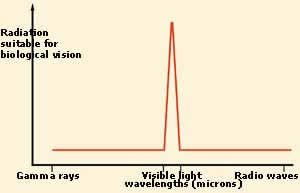
The only rays of light that are suitable
for biological vision have wavelengths that
fall within the range of what is called
"visible light". A large part of the energy
that is emitted by the sun falls in that
range. |
Designing an organic eye that could see other
ranges of the electromagnetic spectrum turns out
to be impossible in a world dominated by carbon-based
life. In Nature's Destiny, Michael Denton explains
this subject in detail and confirms that an organic
eye can only see within the range of visible light.
While other models of eyes that could, in theory,
be designed, none of them would be able to see
different ranges of the spectrum. Denton tells
us why:
UV, X-ray, and
gamma rays are too energetic and are highly destructive,
while infrared and radio waves are too weak to
be detected because they impart so little energy
interacting with matter... And so it would appear
that for several different reasons, the visual
region of the electromagnetic spectrum is the
one region supremely fit for biological vision
and particularly for the high-resolution vertebrate
camera eye of a design and dimension very close
to that of the human eye.73
Pausing to think about everything that has been
said so far, we come to this conclusion: The sun
radiates energy within a narrow band (a band so
narrow that it corresponds to just 1/1025
of the whole electromagnetic spectrum) that has
been carefully chosen. So finely adjusted is this
band that it keeps the world warm, supports the
biological functions of complex life-forms, enables
photosynthesis, and allows the creatures of this
world to see.
The Right Star, the Right
Planet, and the Right Distance
In "The Blue Planet" we compared our world with
the other planets of the solar system and found
that the range of temperatures necessary for life
exists only on Earth. The biggest reason for this
is that the earth is just the right distance from
the sun: the outer planets like Mars, Jupiter,
or Pluto are too cold while the inner planets
Venus and Mercury are too hot.
Those who refuse to admit that there is intentional
design in the distance between Earth and Sun suggest
something like the following:
"The universe is full of stars,
some of them much bigger than the sun and some
of them much smaller. These could very well have
planetary systems of their own. If a star is bigger
than the sun, then the ideal planet for life would
be located at a much greater distance than the
earth is from the sun. For example, a planet in
an orbit around a red giant at the distance of
Pluto could have a temperate climate like our
world has. Such a planet would be just as fit
for life as our earth is."
The claim is invalid in one very important respect
for it ignores the fact that stars of different
masses radiate different types of energy.
The factors that determine the wavelengths of
the energy that a star radiates are its mass and
its surface temperature (the latter of which is
directly related to mass). For example, the sun
radiates near ultraviolet, visible, and near infrared
light because its surface temperature is around
6,000°C. If the sun's mass were a bit bigger,
its surface temperature would be higher; but in
that case, the energy levels of the sun's radiation
would also be higher and the sun would be radiating
much more destructive ultraviolet rays than it
does.
This tells us that any star that is to radiate
light that will support life absolutely must have
a mass close to that of our sun. But if there
are to be life-supporting planets orbiting around
such stars, those planets must be located at distances
not substantially different from that between
the earth and the sun.
In other words, no planet revolving around a
red giant, a blue giant, or any other star whose
mass was substantially different from the sun's
could harbor life. The only source of energy capable
of supporting life is a star like our sun. The
only planetary distance that is suitable for life
is the distance between the earth and the sun.
There is another way of expressing this truth:
The sun and the earth were each created to be
just as they needed to be. And indeed, in the
Qur'an it is revealed that Allah created everything
according to precise calculation:
It is He Who splits the
sky at dawn, and appoints the night as a time
of stillness and the sun and moon as a means of
reckoning. That is what the Almighty, the All-Knowing
has ordained. (Surat al-Anam: 96)
The Harmony of Light and
Atmosphere
Since the beginning of this chapter we have been
talking about the radiation given out by the sun
and how it was specially designed to support life.
There is yet another crucially important factor
that we have not yet touched upon: In order for
this radiation to reach the earth's surface, it
has to pass through the atmosphere.
 Our
sun has a surface temperature of about 6,000°C.
If this temperature were even slightly more
or less, the resulting sunlight would be incapable
of supporting life. Our
sun has a surface temperature of about 6,000°C.
If this temperature were even slightly more
or less, the resulting sunlight would be incapable
of supporting life. |
Sunlight certainly couldn't do us any good if
the atmosphere didn't let it through. But it does;
in fact, our atmosphere is specially designed
to be transparent to this beneficial radiation.
The really interesting thing is not so much that
the atmosphere allows beneficial sunlight to pass
but that sunlight is the only radiation that it
allows through. The atmosphere lets in the visible
and near infrared light that is necessary for
life but it blocks other forms of radiation that
are deadly. This makes the atmosphere an important
filter against the cosmic radiation that reaches
the earth from the sun and from other sources.
Denton has this to say about the matter:
Atmospheric
gases themselves absorb electromagnetic radiation
immediately on either side of the visible and
near infrared... The only region of the spectrum
allowed to pass through the atmosphere over the
entire range of electromagnetic radiation from
radio to gamma rays is the exceedingly narrow
band including the visible and near infrared.
Virtually no gamma, X, ultraviolet, far infrared,
and microwave radiation reaches the surface of
the earth.74
It is impossible to ignore the artfulness of
this design. The sun sends only 1/1025
of the whole range of electromagnetic radiation
that could be sent, that happens to be the range
that is good only for us, and that is the radiation
that the atmosphere lets through! At this point
it's also worth pointing out that nearly all of
the near ultraviolet that the sun radiates gets
trapped by the atmosphere's ozone layer.
 Although
it blocks all other forms of radiation, water
allows visible light to penetrate into its
depth for many meters. Thanks to this, sea
plants are able to perform photosynthesis.
If water did not have this property, the ecological
balance necessary for life on our planet could
not have come into being. Although
it blocks all other forms of radiation, water
allows visible light to penetrate into its
depth for many meters. Thanks to this, sea
plants are able to perform photosynthesis.
If water did not have this property, the ecological
balance necessary for life on our planet could
not have come into being. |
Another point that makes this even more interesting
is that, like air, water also has an extremely
particular sort of transparency: the only radiation
capable of spreading through water is the range
of visible light. Even near infrared radiation,
which penetrates the atmosphere (and thus provides
heat) penetrates only a few millimeters into water.
Because of this, only a few millimeters of the
surface of the world's oceans are heated by radiation
from the sun. That heat is conveyed in stages
to lower levels and as a result of this, below
a particular depth, the temperature of the seawater
is quite similar all over the world. This of course
creates an environment quite suitable for life.
Another interesting point concerning water is
that the different colors of visible light are
able to travel different distances in it. Below
eighteen meters, for example, red light cannot
penetrate while yellow can reach depths of up
to a hundred meters. Blue and green on the other
hand descend to 240 meters. This is an extremely
important design because the light that is particularly
crucial for photosynthesis is the blue and green
portion of the spectrum. Since water allows these
colors to penetrate more deeply than the others,
photosynthesizing plants can live up to 240 meters
beneath the surface.
These are all facts of the utmost importance.
No matter what physical law related to light we
examine, we discover that everything has been
exactly arranged so that life can exist. Commenting
on this situation, Encyclopedia Britannica
admits how extraordinary it all is:
Considering
the importance of visible sunlight for all aspects
of terrestrial life, one can not help being awed
by the dramatically narrow window in the atmosphere
absorption and in the absorption spectrum of water.
75
Conclusion
Materialist philosophy and Darwinism, which takes
materialism as its source, both claim that human
life appeared in the universe by chance and that
it is an "accident" with no purpose whatsoever.
The knowledge that is being gained through advances
in science however is showing that, in every detail
of the universe, there is a design and a plan
whose intention is human life. It is such a design
that, even such a component as light, which we
might never have thought about before, is so clearly
"just right" that one can't help but be amazed.
To try and explain such careful design as "accidental"
is irrational. The fact that all the sun's radiation
is constricted to a narrow band just 1/1025 of
the total electromagnetic spectrum, the fact that
the light necessary for life falls precisely within
that narrow band, the fact that the atmosphere
blocks all other wavelengths of radiation and
admits just these, the fact that water also blocks
all other forms of deadly radiation and permits
the passage only of visible light: Can these really
all be coincidences? Such extraordinary fine-tuning
as this can be explained not by chance but only
by conscious design. This in turn shows us that
the whole universe and all the details of that
universe-including the light of the sun that enables
us to see and keeps us warm-have been specially
created and arranged for us to live.
The conclusion reached by science is a truth
that has been taught to mankind in the Qur'an
for fourteen centuries. Science shows that sunlight
has been created for us, in other words, that
it has been made to be "at our service". In the
Qur'an we are told that "The
sun and moon both run with precision." (Surat
ar-Rahman: 5) Elsewhere it is stated:
Allah is He who created
the heavens and the earth and sends down water
from the sky and by it brings forth fruits as
provision for you. ...He has made the sun and
moon subservient to you holding steady to their
courses, and He has made the night and day subservient
to you. He has given you everything you have asked
Him for. If you tried to number Allah's blessings,
you could never count them. Man is indeed wrongdoing,
ungrateful. (Surah Ibrahim: 32-34) |
